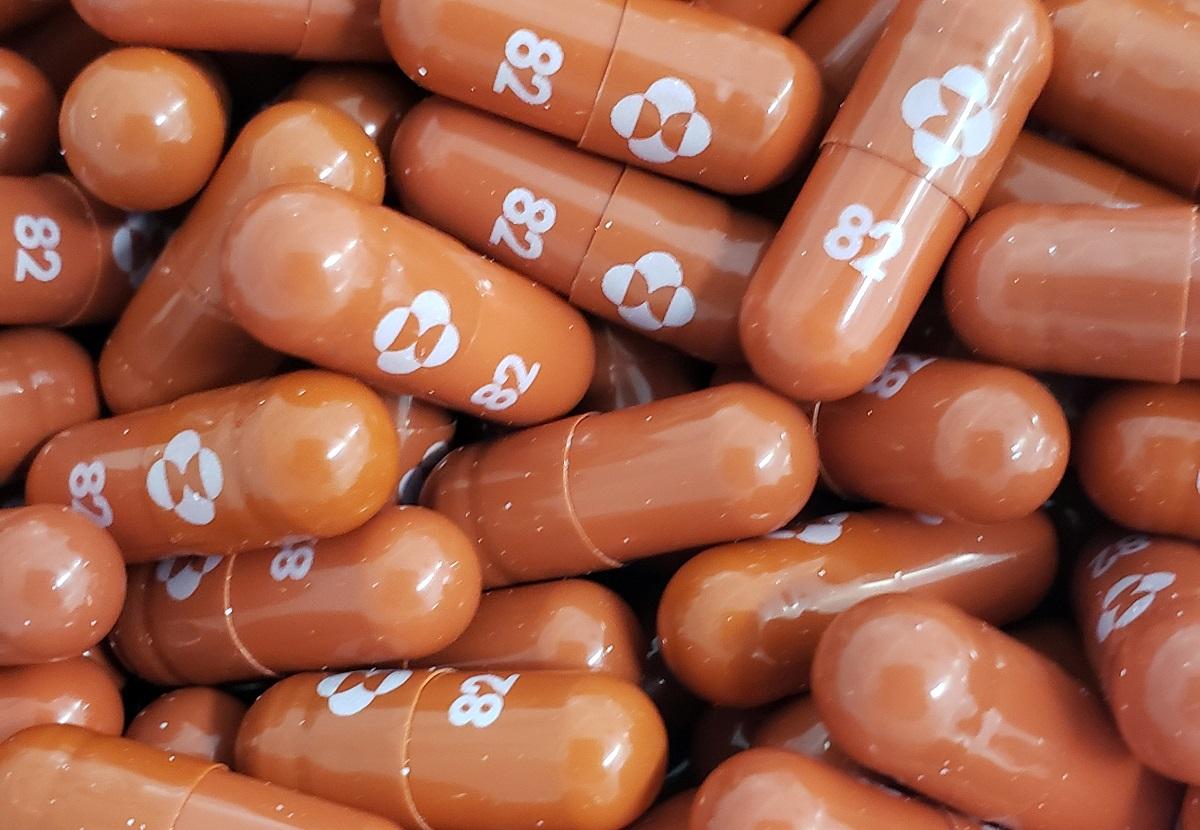
It is up to Merck’s licensed partners to apply for emergency use authority for anti-COVID-19 bill molnupiravir with the Food and Drug Administration in the Philippines, MSD Philippines president Dr. Beaver Tamesis said on Monday.
In an online news forum organized by the Foreign Correspondents Association of the Philippines, Tamesis said MSD had signed licensing agreements which are meant to cover 105 low income to middle income countries.
“Because we’ve signed a voluntary licensing agreement which will cover about 105 lower, middle-income countries. MSD Philippines will no longer be filing for regulatory approval by ourselves,” Tamesis said.
“We will not try for molnupiravir with the FDA. It now becomes incumbent upon our licensing partners… They will be the ones pursuing registration in the Philippines,” he added.
Tamesis said the MDS signed the licensing agreements with seven Indian companies to make molnupiravir more accessible to the public.
“I mentioned earlier that we had already signed voluntary licensing agreements with these five, seven Indian companies. The reason for that is that we wanted to improve access and we wanted to improve the pricing,” Tamesis said.
A health expert previously said molnupiravir may be taken by an individual once he or she is exposed to COVID-19. It is said that the pill decreased by half the chances of acquiring the illness. -NB, GMA News

0 Comments